Articles by Lim Jeong-yeo

Lim Jeong-yeo
-
How far will Samsung Biologics’ shares soar?
Biologics companies are receiving more investor attention than ever due to the COVID-19 pandemic. But the question remains if the sudden buoyance is a bubble that can burst at any moment. Among the publicly traded Korean biologics companies, market watchers are especially eyeing Samsung Biologics, Celltrion and soon-to-be listed SK Biopharmaceuticals. Samsung Biologics shares especially soared past the 800,000 won ($660) after the Seoul Central District Court denied an arrest warrant for Samsu
Industry June 23, 2020

-
SD Biotechnologies expands presence in overseas markets
South Korea’s SD Biotechnologies is rapidly fueling its business overseas through online and offline channels, according to officials. The beauty and health care company boasts six growing brand lines -- skin care brand SNP, standing for “shining,” “nature” and “purity”; colored makeup cosmetics brand Celebeau; urban pollution-tackling dermatologic cosmetic brand hddn=lab; professional barber grooming brand for men M’Solic; organic cotton-covered
Industry June 23, 2020

-
[Herald Interview] Spinning cord blood into stem cell therapies
Cord blood-originated stem cell therapeutics firm Medipost’s CEO Yang Yoon-sun recounts 20 years of her entrepreneurship as being dotted with incessant challenges. Her journey as a professor of pathology at Samsung Medical Center to head of a cord blood stem cell research company at the age of 37 came with prejudices, challenges and pitfalls, but also with encouragement, conviction and immense potential. Cord blood is that inside the umbilical cord that connects the baby and mother
Industry June 22, 2020
![[Herald Interview] Spinning cord blood into stem cell therapies](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/06/16/20200616000193_0.jpg&u=20200623095813)
-
Chong Kun Dang’s bispecific antibody effective in TKI-resistant animal models
South Korea’s Chong Kun Dang presented its latest study results of the bispecific antibody pipeline CKD-702 that simultaneously inhibits c-Met and epidermal growth factor (EGFR) in animals who have grown resistant to existing tyrosine kinase inhibitors, at the online American Association of Cancer Research Annual Meeting 2020 that kicked off Monday. The CKD-702, currently being developed to treat non-small cell lung cancer in Korea, was tested on animals in single administrations to test
Industry June 22, 2020

-
SK Biopharmaceuticals sets IPO price at $40
SK Biopharmaceuticals, a novel drug research and development company specializing in the diseases pertaining to the central nervous system, has set its initial public offering price at 49,000 won ($40) per share, it said Friday. The company announced that this is the top end of its indicative price range, which had suggested the share price would be between 36,000 won and 49,000 won. This puts the total public offering value of SK Biopharmaceuticals at 959.3 billion won, with the market c
Business June 19, 2020

-
Medical AI company Vuno taps Japan through M3
Medical artificial intelligence solution provider Vuno said Friday it plans to tap the Japanese market through Sony’s subsidiary M3. M3 is 33.9 percent owned by Sony and is Japan’s biggest medical data platform company with 280,000 medical professionals enrolled as members. It has accumulated knowledge in the conservative Japanese medical field, and provides a wide range of services including clinical test designing, marketing support for pharmaceutical firms and remote medicine.
Industry June 19, 2020
-
Galaxy S20+ BTS Edition preorders close in 1 hour
“I purple you,” Samsung Galaxy S20+ BTS Edition, is selling like hot cakes. Preorders for Samsung Electronics’ Galaxy S20+ 5G phone and wireless earphones Buds+ BTS Editions, garbed in the K-pop group’s iconic purple and accentuated with tiny purple hearts and BTS logo, closed in an hour since opening in South Korea, Friday. The set is priced at 1.6 million won ($1,320), not a far-off figure from the combined price of a regular S20+ and Buds+, which cost about 1.5 milli
Industry June 19, 2020

-
Psomagen, Cell Biotech sign MOU for microbiome biz
US-based Psomagen and Korean probiotics company Cell Biotech said Friday they will jointly strive for big data-based microbiome research and commercialization. According to the memorandum of understanding, Psomagen and Cell Biotech will research microbial organisms that will show clinical efficacy in health care, as well as develop new probiotics products. Using Psomagen’s big data analysis technology, they will identify potential novel drug materials and develop probiotics products sp
Industry June 19, 2020

-
Drug Ministry revokes Meditoxin license
South Korea’s first botulinum toxin product Meditoxin has been stripped of its license, as of Thursday, in a blow dealt by the Drug Ministry against the company’s use of unauthorized substances in the botulinum toxin products manufactured between 2012 and 2015. Meditoxin, the BTX product accountable for 42 percent of Medytox’s yearly revenue in 2019, had been suspended of production and sales since April 17. It will lose the sales permit altogether from June 25, the Drug Minis
Industry June 18, 2020

-
Chong Kun Dang’s nafamostat drug proceeds to COVID-19 phase 2 trials
Chong Kun Dang said Thursday its anti-coagulant drug Nafabeltan (nafamostat) is to carry out clinical phase 2 trials to be repurposed as a COVID-19 treatment. The company said in a joint press release with Institut Pasteur Korea and Korea Institute of Radiological & Medical Sciences that Nafabeltan has demonstrated strong antiviral qualities against COVID-19 in a cellular-level test. Instiut Pasteur Korea had explored the antiviral qualities of over 3,000 molecules of which it found that
Industry June 18, 2020

-
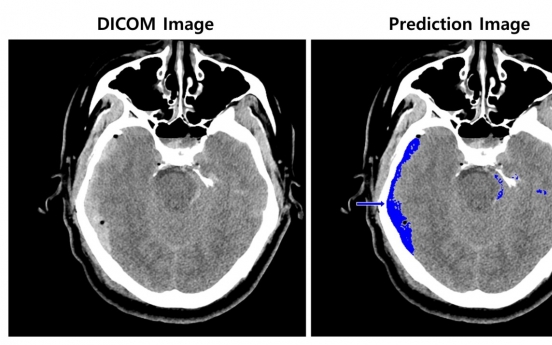
AI will save those who suffer cerebral hemorrhage: SK C&C
It won’t be long before artificial intelligence can help save those who suffer cerebral hemorrhage, according to SK C&C, which is preparing to clinically test its medical device designed for the purpose. The company said Thursday that its AI model acquired the Drug Ministry’s Good Manufacturing Practice certificate, guaranteeing the medical device’s quality control. Following the GMP, SK C&C has submitted its proposed clinical test design to the ministry for tests of
Industry June 18, 2020
-
JW Pharmaceutical to repurpose targeted oncology drug as COVID-19 therapy
JW Pharmaceutical said Wednesday it has applied to patent its anti-cancer pipeline CWP291 as a potential COVID-19 treatment. CWP291 is a Wnt/beta-catenin inhibitor that is under development as a first-in-class treatment for acute myeloid leukemia, multiple myeloma and stomach cancer. The South Korean firm is reviewing the pipeline’s ability to hinder Glucose Regulated Protein 78 (GRP78) manifestation to be applicable as a COVID-19 treatment. GRP78 plays a central role in a tumor&rsqu
Industry June 17, 2020

-
GCMS signs $30m COVID-19 test kit export deal
GCMS, an in-vitro diagnosis service provider under GC Pharma, said Wednesday that it signed three deals worth $30 million in total, to provide COVID-19 test kits to Europe, Middle East and Asia. “Our full line-up of COVID-19 diagnostics test kits, ranging from molecular diagnosis to immunodiagnosis, will add fuel to our exports and this year‘s revenue,” GCMS CEO Ahn Eun-uk said. “Based on an open-innovation strategy, we will continue to bolster our diagnostics busine
Industry June 17, 2020
-
[News Focus] Fanfare for SK Biopharm stock debut, but should you invest?
SK Biopharmaceuticals’ imminent initial public offering on South Korea’s main bourse Kospi has the stock market excited. But is it really the right time to buy? The global coronavirus pandemic has definitely given investors a moment to give a second look at previously-bypassed biologics stocks, and many who have never purchased any stocks are contemplating trying their chances. But as SK Biopharmaceuticals’ CEO Cho Jeong-woo said at the online press event on Monday, just
Industry June 16, 2020
![[News Focus] Fanfare for SK Biopharm stock debut, but should you invest?](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/06/16/20200616000867_0.jpg&u=20200616171922)
-
Celltrion plans 2 types of COVID-19 test kits for overseas markets
Celltrion said Tuesday that it plans to release two types of COVID-19 test kits for approval this month for overseas markets from no later than July. Both tests require just 15 to 20 minutes’ analyzation time, Celltrion said. Celltrion has co-developed point-of-care tests with kit maker BBB. The tests use small machinery that analyzes blood and saliva. They are characterized for their 95 percent high sensitivity, as well as being slightly costly. Celltrion said it will have the point-
Industry June 16, 2020

Most Popular
-
1
Actor Jung Woo-sung admits to being father of model Moon Ga-bi’s child

-
2
Wealthy parents ditch Korean passports to get kids into international school

-
3
Man convicted after binge eating to avoid military service

-
4
First snow to fall in Seoul on Wednesday

-
5
Final push to forge UN treaty on plastic pollution set to begin in Busan

-
6
Korea to hold own memorial for forced labor victims, boycotting Japan’s

-
7
Nvidia CEO signals Samsung’s imminent shipment of AI chips

-
8
Job creation lowest on record among under-30s

-
9
NK troops disguised as 'indigenous' people in Far East for combat against Ukraine: report

-
10
Opposition leader awaits perjury trial ruling




![[Herald Interview] Spinning cord blood into stem cell therapies](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/06/16/20200616000193_0.jpg&u=20200623095813)










![[News Focus] Fanfare for SK Biopharm stock debut, but should you invest?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/06/16/20200616000867_0.jpg&u=20200616171922)










