Articles by Lim Jeong-yeo

Lim Jeong-yeo
-
Genexine’s COVID-19 drug to begin phase 1 trial in Korea
Genexine‘s COVID-19 treatment candidate GX-17 was given the Korean drug authority’s grant to proceed to phase 1 human trials, Friday. GX-17 is a protein drug recombining human IL-7 and hybrid Fc (hyFc) that the company had been developing as an anti-cancer treatment. It is a T-Cell growth factor that proliferates a patient‘s immune cells, by which it slows the progression of an illness and hastens recovery. With the addition of GX-17, South Korea now has 13 treatment resea
Industry Aug. 7, 2020

-
Daewoong fights for continued availability of Jeuveau in US
Daewoong Pharmaceutical on Friday accused the US’ International Trade Commission’s decision to put a 10-year sales ban on its botulinum toxin product of “lacking evidence” and being “biased and distorted.” Daewoong said it has filed a detailed complaint to the USITC for its “significant errors.” Daewoong has been enwrapped in legal battles in Korea and in the US against Medytox for nearly five years over an alleged theft of trade secrets by a for
Industry Aug. 7, 2020

-
Celltrion operating profit rises 118% to it new record
Celltrion posted a record-setting second quarter report on Friday, citing Truxima’s rapid market uptake in the US and increased productivity at its reinforced plant No. 1 in Songdo, Incheon. Celltrion’s second quarter revenue reached 428.8 billion won ($361.7 million), up 82.5 percent on-year, and operating profit hit 181.8 billion won, up 118 percent on-year. Both are the highest numbers Celltrion has reported in any quarter. The company is confident it has ample power to generate
Industry Aug. 7, 2020

-
Enzychem Lifesciences’ COVID-19 drug proceeds to phase 2 trial
Enzychem Lifesciences’ COVID-19 treatment candidate EC-18 was granted by the US’ Food and Drug Administration to carry out phase 2 clinical trial on confirmed patients, the company said Friday. In this phase 2 clinical trial, Enzychem Lifesciences will confirm the therapeutic effects of EC-18 for acute respiratory distress syndrome prevalent in COVID-19 patients with pneumonia. EC-18 is a novel drug candidate that had been in phase 2 clinical trials for chemoradiation-induced oral
Industry Aug. 7, 2020

-
[Herald Interview] Arvelle was founded to in-license cenobamate: CEO
Arvelle Therapeutics, founded in 2019, is a Switzerland-based biopharmaceutical company that focuses on innovative central nervous system drugs. The company’s noticeable idiosyncrasy? It has only one pipeline, which happens to be South Korean SK Biopharmaceuticals’ cenobamate, a drug used to treat partial-onset seizures in adults. “Arvelle was founded to in-license cenobamate,” said Mark Altemeyer, CEO of Arvelle, in an exclusive interview with The Korea Herald held acr
Industry Aug. 6, 2020
![[Herald Interview] Arvelle was founded to in-license cenobamate: CEO](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/08/04/20200804000076_0.jpg&u=20200806174359)
-
Samsung Biologics launches S-CHOice cell line expression technology
Samsung Biologics on Tuesday launched a proprietary cell line expression technology called S-CHOice, intent to improve its contract development services by shortening the drug development period for clients and lowering their drug product costs. Through an online ceremony streamed via YouTube, the company’s vice president of contract development business, Yang Eun-young, and director of cell line development, John Gill, introduced S-CHOice, which the company will offer to “all clie
Industry Aug. 5, 2020

-
Hanmi’s once returned pipeline finds new partner in US
Hanmi Pharmaceutical licensed out HM12525A to American firm MSD on Tuesday as a nonalcoholic fatty liver treatment candidate -- a promising turn of events after the pipeline was returned by Janssen last year. HM12525A, variably known as “LAPSGLP/Glucagon receptor dual agonist” and “efinopegdutide,” was first licensed out to Belgium’s Janssen in 2015 as a potential treatment for obesity and diabetes. Janssen later determined in 2019 to cease the research and return
Industry Aug. 4, 2020

-
Bioneer to supply Colombia with W5.6b worth of COVID-19 kits
Bioneer signed a deal worth 5.6 billion won ($4.7 million) to supply the South American country of Colombia with COVID-19 test kits and sequencing machine, the company said Tuesday. Bioneer has the capability to provide both COVID-19 test kits and the sequencing machine to read the kits. It asserts to be the only Korean firm with such total-solution capacity. Under the new deal, Bioneer will provide 5 billion won worth of COVID-19 kits and 600 million won worth of machinery to Colombia in the
Industry Aug. 4, 2020

-
[From the Scene] Inside the world’s biggest biologics plant
This article was written after a one-on-one tour of Samsung Biologics’ plants. -- Ed. If this era insists that there be “photos or it didn’t happen,” then this trip to Samsung Biologics’ plants may as well have not taken place. All Samsung plants are under strict security protocols, meaning phones only pass the security gate once tape is placed over both the front and rear cameras. There is also no Wi-Fi for visitors. So, since no selfies and no candid videos c
Industry Aug. 3, 2020
![[From the Scene] Inside the world’s biggest biologics plant](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/08/03/20200803000630_0.jpg&u=20200803203838)
-
Chong Kun Dang’s net profit rises 100% on-year in Q2
Chong Kun Dang’s second quarter net profit rose 100 percent on-year due to decreased spending on marketing amid COVID-19 social distancing, and solid sales of its chronic illness treatments. Hi Investment & Securities said Monday it is upward-adjusting prospects for Chong Kun Dang by 26.9 percent from previous 130,000 won ($109) to 165,000 won. Shinyoung Securities set the prospect slightly higher at 170,000 won and reinforced the opinion to buy. Chong Kun Dang’s performance wa
Industry Aug. 3, 2020

-
LG Chem anticipates smooth resolution with SK Innovation
LG Chem is talking with SK Innovation to reach a settlement regarding a trade secret violation suit against the latter, and anticipates a resolution before October, the firm said Friday. “We can settle the case through negotiations before the final decision in October, should the settlement be on a reasonable level based on objective facts,” said LG Chem during a quarterly conference call with investors. The US International Trade Commission has made a default judgement in favor of
Industry July 31, 2020

-
LG Chem’s EV batteries drive best-ever performance in Q2
LG Chem posted a second quarter revenue of 6.9 trillion won ($5.8 billion) and an operating profit of 571.6 billion won on Friday, marking an earnings surprise on the back of its electric vehicle batteries. The revenue is a 2.3 percent on-year increase and the operating profit a 131.5 percent jump, the best performance LG Chem has shown in any quarter. LG Chem credited improved productivity at its Poland plant -- arising from an increased number of quality products among all manufactured goo
Industry July 31, 2020

-
LG Electronics beats Whirlpool in Q2 earnings
If the semiconductor business perks up Samsung Electronics’ bottom line, it’s home appliance that does it for crosstown rival LG Electronics. LG Electronics posted an overall slide in its second-quarter earnings, but still came out resilient, posting earnings bigger than US’ Whirlpool. The company reported a 17.9 percent fall in revenue and 24.1 percent drop in operating profit, at 12.8 trillion won ($10.7 billion) and 495.4 billion won, respectively. However, in the consume
Industry July 30, 2020

-
Celltrion’s COVID-19 therapy to begin phase 1 trial in UK
Celltrion’s COVID-19 antibody therapy candidate CT-P59 was given the approval to carry out clinical phase 1 trial in the UK, the company said Thursday. A clinical phase 1 trial is conducted on a small group of relatively healthy people, to test the drug candidate’s preliminary indications in human body, including its effectiveness, toxicity and any possible side effects. Celltrion said it is now recruiting eligible people for the trial. After this trial will follow Clinical pha
Industry July 30, 2020

-
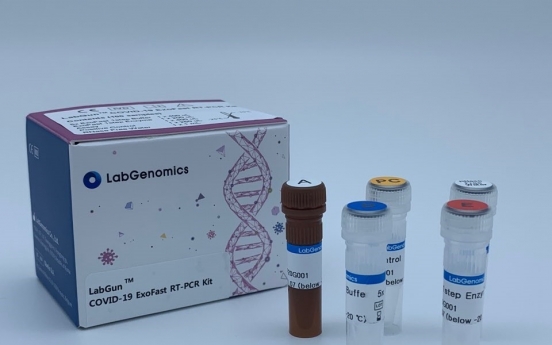
LabGenomics to supply advanced COVID-19 test kits to India
South Korea’s LabGenomics, which focuses on molecular diagnostics, said Thursday it has bagged a deal to supply advanced COVID-19 test kits to Siemens Healthineers in India. According to the company, it has signed a 5.8 billion won ($4.8 million) contract with the Indian medical technology firm to supply real-time polymerase chain reaction test kits for COVID-19 that can detect SARS CoV-2 virus infection in 35 minutes. This deal is worth 17.5 percent of LabGenomics’ yearly revenue
Industry July 30, 2020
Most Popular
-
1
Actor Jung Woo-sung admits to being father of model Moon Ga-bi’s child

-
2
Wealthy parents ditch Korean passports to get kids into international school

-
3
Man convicted after binge eating to avoid military service

-
4
First snow to fall in Seoul on Wednesday

-
5
Final push to forge UN treaty on plastic pollution set to begin in Busan

-
6
Korea to hold own memorial for forced labor victims, boycotting Japan’s

-
7
Nvidia CEO signals Samsung’s imminent shipment of AI chips

-
8
Job creation lowest on record among under-30s

-
9
NK troops disguised as 'indigenous' people in Far East for combat against Ukraine: report

-
10
Opposition leader awaits perjury trial ruling






![[Herald Interview] Arvelle was founded to in-license cenobamate: CEO](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/08/04/20200804000076_0.jpg&u=20200806174359)



![[From the Scene] Inside the world’s biggest biologics plant](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2020/08/03/20200803000630_0.jpg&u=20200803203838)















