Most Popular
-
1
Now is no time to add pressure on businesses: top executives

-
2
CJ CheilJedang to spur overseas growth with new Hungary, US plants

-
3
Seoul to host winter festival from Dec. 13

-
4
Blackpink's solo journeys: Complementary paths, not competition

-
5
Nationwide rail disruptions feared as union plans strike from Dec. 5

-
6
N. Korea, Russia court softer image: From animal diplomacy to tourism

-
7
Smugglers caught disguising 230 tons of Chinese black beans as diesel exhaust fluid

-
8
[Today’s K-pop] Blackpink’s Jennie, Lisa invited to Coachella as solo acts
![[Today’s K-pop] Blackpink’s Jennie, Lisa invited to Coachella as solo acts](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/21/20241121050099_0.jpg&u=20241121172748)
-
9
Actor Song Joong-ki welcomes second child in Rome

-
10
Main opposition pushes to ease, not postpone, tax on crypto gains

-
Korean biosimilars make inroads into Europe
[THE INVESTOR] Propelled by Samsung Bioepis and Celltrion, South Korean biopharmaceutical companies are gaining a foothold in Europe with their biosimilars, cheaper copycat versions of biologics. Celltrion’s Remsima -- biosimilar replication of rheumatoid arthritis treatment drug Remicade -- and Samsung Bioepis’ Benepali -- biosimilar copy of Amgen’s rheumatoid arthritis treatment Enbrel – along with Flixabi, another Remicade biosimilar, are already selling in Europe, one of the world’ largest m
July 19, 2016

-
Boyrung Biopharma launches 5-year cord blood research project
[THE INVESTOR] Boryung Biopharma is to research immune cell treatments using umbilical cord blood in collaboration with Seoul St. Mary’s Hospital.The company said on July 14, that it signed an agreement with the hospital to conduct research in a 5-year project aimed at developing a range of treatments for immune cells. By Choi He-suk (cheesuk@heraldcorp.com)
July 15, 2016
-
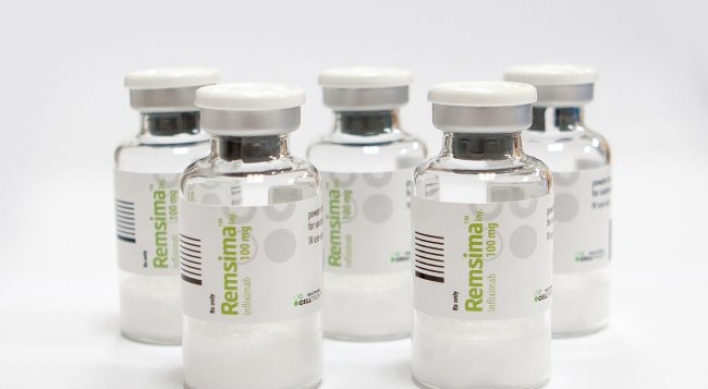
Celltrion’s Remsima bolsters presence in Europe
[THE INVESTOR] South Korean biopharmaceutical company Celltrion has increased its market presence in Europe with biosimilar Remsima, the company said on July 14. Celltrion’s Remsima -- biosimilar replication of the world’s third best-selling rheumatoid arthritis treatment drug Remicade, or Infliximab -- has captured a 30 percent share of the overall Infliximab market in Europe, according to IMS Health, a market data provider. Celltrion's biosimilar RemsimaThe latecomer Remsima began selling in E
July 14, 2016
-
Kolon Life Science seeks approval for degenerative arthritis drug
[THE INVESTOR] Kolon Life Science has filed for government approval for a drug it developed to treat degenerative arthritis, the company said on July 11. After 17 years of research, the pharmaceutical company introduced Invossa, Korea’s first gene therapy for degenerative arthritis. The new drug injects healthy human cartilage cells into the space between the bones to alleviate pain and treat the major symptoms. It can also be an alternative for surgical treatment, according to Kolon Life Scienc
July 12, 2016

-
Human embryo research to resume in S. Korea
[THE INVESTOR] South Korea’s Cha University has been granted conditional permit to carry out research using human embryos, the Ministry of Health and Welfare said on July 11. The research will be carried out using somatic cell nuclear transfer method. The method transfers the nucleus, which contains the majority of human genetic information, from a somatic cell into an ovum whose nucleus has been removed. Pixabay.comThis is the first time research using human embryos has been permitted in Korea
July 11, 2016

-
Green Cross Labcell files patents for anti-cancer cells
[THE INVESTOR] Korean pharma firm Green Cross Labcell has applied for international patent protection for its natural killer cells that treat cancer patients, the company said on July 8.The Green Cross affiliate had extracted cord blood from healthy donors to generate natural killer cells, a type of white blood cells in the immune system that attacks infections and potentially cancer-causing cell. Institute of Cell Therapy at the Green Cross LabcellThe cord blood-derived natural killer cells, wh
July 8, 2016

-
S. Korea hopes to triple cosmetics exports
[THE INVESTOR] South Korean government aims to boost cosmetics exports to US$ 7 billion by 2018 through deregulation and by supporting export activities. Last year, Korea’s cosmetics exports came in at US$ 2.6 billion. The plans were revealed at the pan-government conference on boosting exports and investment held in Seoul on July 7. As part of the measures, cosmetics producers, universities and research organizations will be allowed to submit products they develop for government certification r
July 7, 2016

-
Price of biosimilar, new drugs to increase
[THE INVESTOR] The price of biosimilars and new drugs that are covered under the national health insurance will increase from October, government plans showed on July 7. The price of biosimilar drugs will be priced up to 80 percent of the branded drugs, a 10 percent increase from the current 70 percent rate. The Health and Welfare Ministry unveiled a set of measures to foster the local pharmaceutical industry in a trade and investment promotion meeting chaired by President Park Geun-hye on July
July 7, 2016
-
Korean gov’t to boost support for medical institutions’ overseas expansion
[THE INVESTOR] The government will draw up support measures for local medical institutions that enter overseas markets, and allow insurance schemes for foreign medical tourists, the government revealed on July 5. The plans were included in the government’s strategy for boosting Korea’s service industry. Support for medical institutions’ overseas expansion will include funding and tax benefits. As part of the plans for luring medical tourists to Korea, insurance companies will be allowed to sell
July 5, 2016
-
Big Korean pharma firms launch venture capital biz
[THE INVESTOR] A handful of Korean pharmaceutical companies are scurrying to set up venture capital firms to find new revenue sources by investing in smaller firms in need of capital. Hanmi Pharm announced on July 4 that it has founded a 10 billion won (US$ 8.7 million) investment company that will invest into biotech and pharmaceutical fields. “We will contribute to create an open innovation ecosystem by focusing on discovering new drug candidates and pharmaceutical and biotech start-ups,” said
July 5, 2016

-
Bridge Biotherapeutics wins W11.5b investment
[THE INVESTOR] Bridge Biotherapeutics announced on July 4 that it has received 11.5 billion won (US$ 10 million) investment from local venture capital firms including LB Investment and KB Investment. Founded last year, Bridge Biotherapeutics is a local developer of biopharmaceuticals that focuses on commercializing compounds developed or discovered by a third party. The company is currently working on BBT-401, anti-inflammatory agent discovered by a research team led by Sungkyunkwan University’s
July 4, 2016
-
Hanmi Pharm launches biopharma venture firm
[THE INVESTOR] Hanmi Pharm announced on July 4 that it has founded a 10 billion won (US$ 8.7 million) investment company that will invest in biotech and pharmaceutical fields. The new company, named Hanmi Ventures, will be headed by Hanmi IT CEO Lim Jong-hoon, and the capital was raised from Hanmi Pharm chairman Lim Sung-ki and the company’s affiliates. Hanmi Ventures plans to initially focus on investing in pharmaceutical and biotech related fields, and Hanmi Science and Hanmi Pharm will take o
July 4, 2016









![[Today’s K-pop] Blackpink’s Jennie, Lisa invited to Coachella as solo acts](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/21/20241121050099_0.jpg&u=20241121172748)













