Altamira Therapeutics Reports Positive Top-Line Data from Bentrio Clinical Trial in Seasonal Allergic Rhinitis
By PRNEWSWIREPublished : May 26, 2023 - 10:10
- Bentrio® meets primary efficacy endpoint in NASAR clinical trial in seasonal allergic rhinitis
- Clinically relevant and statistically significant improvement in Total Nasal Symptom Score over saline nasal spray control (p = 0.012)
- Bentrio efficacy and tolerability rated as "good" or "very good" by 63.5% and 73.5% of study participants
- NASAR concludes Altamira's clinical development program for Bentrio in allergic rhinitis
SHANGHAI, May 26, 2023 /PRNewswire/ -- Altamira Therapeutics Ltd. (Nasdaq: CYTO), a company dedicated to developing therapeutics that address important unmet medical needs, today announced positive and statistically significant top-line results from the randomized controlled NASAR clinical trial evaluating its Bentrio nasal spray in patients with seasonal allergic rhinitis (SAR). Bentrio nasal spray is formulated as a drug-free and preservative-free gel emulsion designed to help protect against airborne allergens such as pollen or house dust mites.
The NASAR trial enrolled 100 SAR patients in Australia who were randomized at a 1:1 ratio to receive either Bentrio or saline nasal spray for two weeks via self-administration three times per day, or as needed. For eligibility, patients had to have a baseline reflective Total Nasal Symptom Score (rTNSS) of at least 5 points out of 12, referring to the worst level of nasal congestion, sneezing, nasal itching, and rhinorrhea (runny nose) within the past 24 hours averaged over a one-week treatment-free run-in period. The primary efficacy endpoint was defined as the difference in the average rTNSS over the subsequent 2-week treatment period between Bentrio and saline nasal spray, the current standard of care in drug-free SAR management. The change in mean rTNSS over two weeks is generally accepted as a primary efficacy endpoint for SAR trials and is also recommended by the FDA.
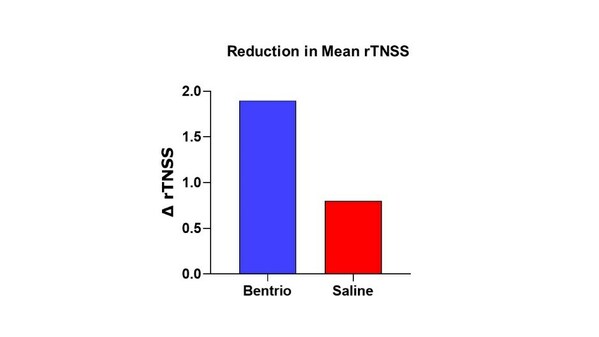
Graph above compares reduction in mean reflective Total Nasal Symptom Score (rTNSS) with Bentrio vs. saline nasal spray control over two weeks of treatment from baseline
The rTNSS decreased in the Bentrio group from 6.9 points in the pre-treatment period to an average of 5.0 points over the 14-day treatment period (i.e. -1.9 points), while the saline spray group showed a decrease from 6.9 to 6.2 points (i.e. -0.8 points) (see 'Mean rTNSS' graph). The reduction in nasal symptoms conferred by Bentrio was thus 2.5 times larger than with saline nasal spray. The difference in rTNSS reduction of 1.1 points in favor of Bentrio was statistically significant in the ANCOVA model (LSmeans; p = 0.012; 95% confidence interval -2.0 to -0.3), and the study thus met the primary efficacy endpoint.
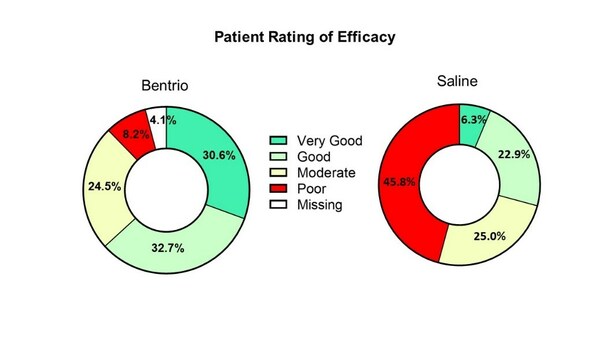
Graph above compares patient rating of treatment efficacy with Bentrio vs. saline nasal spray control after two weeks of treatment
The treatment effect shown with Bentrio was well above the minimal clinically important difference of 0.28 points. 63.3% of Bentrio-treated study participants rated treatment efficacy as either good or very good vs. 29.2% of saline-treated participants (see 'Patient Rating' graph). Among the latter, 45.8% reported efficacy as poor vs. only 8.2% in the Bentrio group. 73.5% of Bentrio-treated study participants rated tolerability of the treatment as either good or very good vs. 85.5% of saline-treated participants. Among the latter, 10.4% reported tolerability as poor vs. only 6.1% in the Bentrio group.
"We are thrilled with the strong outcomes from the NASAR trial, which we obtained under real-life conditions during the allergy season in Australia," commented Thomas Meyer, Altamira Therapeutics' founder, Chairman, and CEO. "The large magnitude of the reduction of nasal symptoms demonstrates that effective protection against seasonal allergic rhinitis is very well possible with a drug-free treatment. There are many patients who do not respond well to medicated nasal sprays either for lack of efficacy or due to tolerability issues with preservatives or other ingredients. I would like to extend my sincere appreciation to all the patients and investigators involved in the NASAR trial who helped us to reach this important clinical milestone.
"Further, the NASAR results show for Bentrio a statistically significant and clinically highly relevant improvement in efficacy over the current standard of care saline nasal spray treatment," Mr. Meyer added. "Whereas a saline nasal spray aims to rinse out allergen particles, Bentrio forms a thin protective layer which prevents contact of those particles with the nasal mucosa and helps to remove them through natural mucociliary clearance. As already demonstrated in a previous trial, Bentrio stays for about 3.5 hours within the nasal cavity where it can exert its protective effects. In contrast, saline spray is present for only about one hour and provides narrower distribution and less coverage within the nasal cavity.
"With the completion of the NASAR trial, Altamira has concluded its clinical development of Bentrio in allergic rhinitis management. In 2023, the US sales of over-the-counter allergy remedies are estimated to reach close to $4 billion which represents a major opportunity in the non-medicated, preservative-free treatments category. Together with its marketing and distribution partners, Altamira is looking forward to making Bentrio available as an effective and safe treatment option to help patients deal with the daily burden and discomfort associated with allergic rhinitis."
"We are pleased to see our partner, Altamira Therapeutics, has achieved this significant milestone for the Bentrio clinical study. Based on the positive top-line results, we are excited about the potential of Bentrio to address the urgent global need for a novel non-medicated, preservative-free treatment for allergic rhinitis patients." said Mark G. Lotter, CEO and Co-Founder of Nuance Pharma. "We look forward to a successful and strong partnership with Altamira, while delivering this innovative therapy to meet the high unmet medical need in further countries within the APAC region."
In March 2022, Nuance Pharma and Altamira Therapeutics entered into an exclusive licensing and distribution agreement for Bentrio in mainland China, Hong Kong, Macau and South Korea. In November 2022, Nuance Pharma announced to launch Bentrio nasal spray in Hong Kong, China.
About Bentrio
Bentrio is an OTC drug-free nasal spray for personal protection against airborne allergens and, where approved, against airborne viruses. Upon application into the nose, Bentrio forms a protective gel layer on the nasal mucosa. This thin film is designed to prevent the contact of allergens (or virus particles) with cells; in addition, the composition serves to bind such particles and help with their discharge. Together, this is designed to promote alleviation of allergic symptoms (or mitigate upper respiratory tract viral infections). For more info, visit: https://www.aurismedical.com/legacy-programs/bentrio
About Altamira Therapeutics
Altamira Therapeutics (Nasdaq: CYTO) is dedicated to developing and commercializing RNA delivery technology for extrahepatic targets (OligoPhore™ / SemaPhore™ platforms). The Company currently has two flagship siRNA programs in preclinical development beyond in vivo proof of concept: AM-401 for KRAS driven cancer and AM-411 for rheumatoid arthritis. The versatile delivery platform is also suited for mRNA and other types of RNA therapeutics and is planned to be leveraged via out-licensing to pharma or biotech companies. In addition, Altamira is in the process of divesting and/or out-licensing its legacy assets in allergology and viral infection (Bentrio® OTC nasal spray; commercial) and inner ear therapeutics (AM-125 nasal spray for vertigo; post Phase 2; Keyzilen® and Sonsuvi® for tinnitus and hearing loss; Phase 3). Founded in 2003, Altamira is headquartered in Hamilton, Bermuda, with its main operations in Basel, Switzerland. For more information, visit: https://altamiratherapeutics.com/
About Nuance Pharma
Nuance Pharma is an innovation focused biopharmaceutical company, with both late-stage clinical pipeline and commercial stage asset portfolio. Focusing on specialty care, Nuance has established a differentiated combination of commercialized assets and innovative pipeline across respiratory, pain management, emergency care and iron deficiency anemia.
With the mission to address critical unmet medical needs in Asia Pacific, Nuance deploys the Dual Wheel model that develops a global leading innovative pipeline, while maintaining a self-sustainable commercial operation in both China and Asia as a region. For more information, please visit www.nuancepharma.com.
Forward-Looking Statement
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of our control, that could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning our plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. We undertake no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.







![[Weekender] Korea's traditional sauce culture gains global recognition](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/21/20241121050153_0.jpg&u=20241123224317)









![[More than APT] Residents, architects together design homes](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/11/24/20241124050036_0.jpg&u=)
