Sputnik V demonstrates strong protection against Omicron variant, with over 2 times higher virus neutralizing activity compared to the Pfizer vaccine according to a unique independent comparative study conducted by the Spallanzani Institute in Italy
ByPublished : Jan. 20, 2022 - 20:20

Previous studies also demonstrated additional significant strengthening of protection against Omicron by Sputnik Light booster, which can also be a universal booster to other vaccines to strengthen and lengthen their protection against Omicron.
MOSCOW, Jan. 20, 2022 /PRNewswire/ --
- A unique comparative study conducted at the Italian Spallanzani Institute, the leading Italian research institute for infectious diseases, by a joint Italian-Russian team of researchers representing the Institute and the Gamaleya Center shows that the Sputnik V coronavirus vaccine demonstrates more than 2 times higher titers of virus neutralizing antibodies to Omicron (B.1.1.529) variant than 2 doses of Pfizer vaccine (2.1 times higher in total and 2.6 times higher 3 months after vaccination).
o The study was conducted in the equal laboratory conditions in the Spallanzani Institute in Italy on comparable sera samples from individuals vaccinated with Sputnik V and Pfizer with a similar level of IgG antibodies and virus neutralizing activity (VNA) against Wuhan variant.
o Sputnik V shows significantly smaller (2.6 times) reduction of virus neutralizing activity against Omicron as compared to reference Wuhan variant than Pfizer vaccine (8.1-fold reduction for Sputnik V in contrast to 21.4-fold reduction for Pfizer vaccine).
- The study demonstrates that Sputnik V neutralizes the Omicron variant by inducing robust antibody response associated with high levels of protection.
o Among the top quartile of individuals with high RBD-specific IgG antibodies, 100% of those vaccinated with Sputnik V were able to neutralize Omicron variant in comparison to 83.3% of individuals vaccinated with Pfizer.
o Among all samples, 74.2% of Sputnik V-vaccinated sera were able to neutralize Omicron vs 56.9% for Pfizer-vaccinated.
- - The study discusses several reasons for Sputnik V eliciting stronger virus neutralizing antibodies against Omicron, including:
o Sputnik V develops a wider pool of antibodies to different epitopes in contrast to Pfizer vaccine, which utilizes the spike protein in a proline-stabilized form directed mainly to the specific epitopes, which were highly affected by the mutations in the Omicron variant;
o Heterologous prime-boost vaccination regimen of Sputnik scheme;
o Better mimicking of adenoviral vaccine platform of the infection.
- The data supports the results of the recent laboratory study by the Gamaleya Center published in MedRxiv[i] demonstrating that Sputnik V induces robust neutralizing antibody response to Omicron variant, which is further strengthened by Sputnik Light booster:
o As 80% of epitopes in the spike protein recognized by CD8+ T cells are not affected by the mutations in the Omicron variant, Sputnik V elicits strong and long-lasting T-cell response and is expected to provide durable protection against severe disease and hospitalization caused by Omicron.
o Sputnik Light as a booster significantly increases virus neutralizing activity against Omicron, which is comparable to titers observed after Sputnik V against wild-type virus, associated with high levels of protection.
- The Sputnik team stands for the open and transparent comparison of different vaccines and has initiated partnerships with other vaccine producers to conduct joint studies in a number of countries.
o In particular, a "mix & match" trial of a combination of Sputnik Light with vaccines produced by AstraZeneca, Sinopharm, Moderna and Cansino, conducted in 5 provinces in Argentina has demonstrated that Sputnik Light induces stronger antibody and T-cell response as compared to homologous regimen (two shots of the same vaccine).
o Each "vaccine cocktail" combination with Sputnik Light provided a higher antibody titer on the 14th day after administering a second dose when compared to original homogenous (same vaccine as first and second dose) regimens of each of the vaccines.
- Sputnik Light is a universal booster to other vaccines thanks to the optimal configuration of Sputnik vaccine's adenoviral platform which provides better protection against Omicron and other mutations as demonstrated in multiple studies.
- Heterologous boosting with Sputnik Light has proven to be one of the best solutions to prolong the protection period of other vaccines. The Sputnik team urges immediate global, open comparative studies on Sputnik Light and other boosters to COVID vaccines. Any efforts to deter these comparative "mix & match" studies delay the end of the pandemic as the advantages of this most efficient approach will not be utilized.
- Sputnik V has been authorized in 71 countries with a total population of over 4 billion people, and Sputnik Light in more than 30 countries. Sputnik V and Sputnik Light have been developed using a safe technology that has been widely studied for over 30 years and have not been associated with rare serious side effects such as myocarditis or pericarditis. The highest safety and efficacy of Sputnik V and Sputnik Light was demonstrated in more than 30 studies and real-world data publications from more than 10 countries.
- Sputnik V and Sputnik Light can be stored in a conventional refrigerator at +2 +8ºC for 6 months, making them available globally, including in remote territories, without any need to invest in additional cold-chain infrastructure.
The Gamaleya National Research Center of Epidemiology and Microbiology (Gamaleya Center) and the Russian Direct Investment Fund (RDIF, Russia's sovereign wealth fund, investor in Sputnik V and Sputnik Light coronavirus vaccines), today announced a unique independent comparative study conducted at the National Institute for Infectious Diseases Lazzaro Spallanzani (Italy) by a joint team of researchers of the Institute and the Gamaleya Center showing that 2 doses of Sputnik V provide more than 2 times higher geometric mean titers (GMT) of virus neutralizing antibodies to the Omicron variant of COVID than 2 doses of Pfizer vaccine (2.1 times higher in total and 2.6 times higher 3 months after vaccination).
An article by a team of 12 Italian and 9 Russian scientists led by Francesco Vaia, Director of the Spallanzani Institute and Alexander Gintsburg, Director of the Gamaleya Center, has been published in medRxiv (the preprint server for health sciences) and is available at:
https://www.medrxiv.org/content/10.1101/2022.01.15.22269335v1
The study was conducted in Spallanzani Institute in the equal laboratory conditions at the Italian Spallanzani Institute on comparable groups of sera from individuals vaccinated with Sputnik V and Pfizer, with no statistically significant difference in neutralizing activity against Wuhan variant.
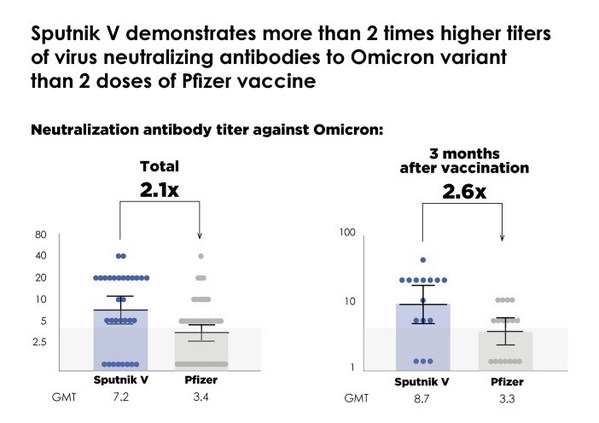
Sputnik V demonstrates more than 2 times higher titers of virus neutralizing antibodies to Omicron variant than 2 doses of Pfizer vaccine
The advantages of Sputnik V are the use of native S glycoprotein (spike protein without proline-stabilization and other modifications) and the use of a heterologous prime-boost vaccination regimen. The Pfizer vaccine utilizes the spike protein in a proline-stabilized form in contrast to Sputnik V. Proline-stabilization and other modifications may move an immune response predominantly to the actively mutating receptor-binding domain (RBD) of spike protein. In the Omicron variant, a substantial number of mutations were registered precisely in RBD, which is why such a significant drop in neutralizing activity against this variant may be observed in the sera of Pfizer-vaccinated.
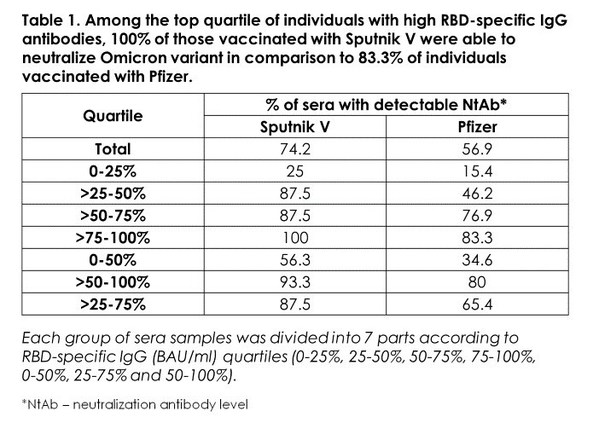
Table 1. Among the top quartile of individuals with high RBD-specific IgG antibodies, 100% of those vaccinated with Sputnik V were able to neutralize Omicron variant in comparison to 83,3% of individuals vaccinated with Pfizer
Boosting with Sputnik Light as part of the "mix & match" approach may help address the lower efficacy of mRNA vaccines against Omicron as well as the documented, quickly waning, efficacy of mRNA vaccines against COVID-19. Partnerships between adenoviral and mRNA vaccines could provide for stronger protection against Omicron and other variants.
Based on the data collected by the Spallanzani Institute and results of previous studies, heterologous ("mix & match") boosting with Sputnik Light is the best solution to increase other vaccines' efficacy and extend the booster protection period as optimal adenoviral platform configuration provides better protection against Omicron and other mutations:
a. Sputnik Light as a booster significantly increases virus neutralizing activity against Omicron, which is comparable to titers observed after Sputnik V against wild-type virus, associated with high levels of protection. The study results were summarized in an article available at:
www.medrxiv.org/content/10.1101/2021.12.17.21267976v1
b. Sputnik Light has already shown strong results as a booster in "mix & match" trials in Argentina. A combination of Sputnik Light with vaccines produced by AstraZeneca, Sinopharm, Moderna and Cansino, conducted in 5 provinces (City and Province of Buenos Aires, as well as Córdoba, La Rioja and San Luis) has demonstrated that Sputnik Light induces stronger antibody and T-cell response as compared to a homologous regimen (two shots of the same vaccine). Each "vaccine cocktail" combination with Sputnik Light provided higher antibody titer on the 14th day after administering a second dose when compared to original homologous (same vaccine as first and second dose) regimens of each of the vaccines.
c. As duration of vaccine protection is key to avoid frequent boosting, authors of another study in Argentina have noted that protection against coronavirus remains stable following vaccination with the Russian Sputnik V vaccine as a consequence of antibody maturation, resulting in improved potency of antibodies to viral escape mutations:
https://www.medrxiv.org/content/10.1101/2021.08.22.21262186v1
d. The study conducted by a number of highly respected institutes in the US (Beth Israel Deaconess Medical Center, Harvard University, Ragon Institute of MGH, MIT and University of North Carolina) demonstrated that boosting Pfizer vaсcine with Ad26 vector produces optimal durable protection against Omicron with 4 times higher increase in Omicron-specific T-cells and 2.4 times in neutralizing antibody titers vs Pfizer booster. Sputnik Light is based on Ad26 vector and is the universal booster for other vaccines vs all mutations:
https://www.medrxiv.org/content/10.1101/2021.12.02.21267198v2
e. US study on 168 mln people showed that one-shot COVID vaccine based on Ad26 is superior against infections and hospitalizations to quickly waning two-shot mRNA vaccines. In the 6th month after vaccination, Pfizer protection against hospitalization waned 4 times, while there was no evidence of waning protection against hospitalization for Ad26 vector:
https://www.medrxiv.org/content/10.1101/2022.01.05.22268648v1
f. Another study conducted on almost 500,000 health care workers in South Africa demonstrated 85% efficacy of Ad26 booster against hospitalization caused by Omicron variant:
https://www.medrxiv.org/content/10.1101/2021.12.28.21268436v1.full-text
Partnership among vaccine manufacturers through heterologous ("mix & match") boosting by other vaccines is needed to address the quickly waning efficacy of mRNA vaccines against COVID-19, which was documented in multiple studies:
a. The study in US among 65+ years old population demonstrated the decrease in mRNA vaccine effectiveness against Delta accelerated after month 4, reaching a low of approximately 20% in months 5 through 7:
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2787183
b. According to Swedish data, the Pfizer vaccine's efficacy against Delta strain is falling to below 30% after 6 months:
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3949410
c. The UK Health Security Agency said those who had received three doses of Pfizer's vaccine saw their protection against symptomatic illness caused by Omicron variant drop to 45% within 10 weeks:
https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings
Alexander Gintsburg, Director of the National Research Center for Epidemiology and Microbiology, said:
"The joint study by the Gamaleya Center and Spallanzani Institute confirmed the results obtained in our separate study published in December 2021. The hard scientific data proves Sputnik V has higher virus neutralizing activity against Omicron as compared to other vaccines and will play a major role in the global fight against this new contagious variant."
Denis Logunov, Deputy Director of the National Research Center for Epidemiology and Microbiology, noted:
"The Spallanzani Institute is one of the leading European research centers and our joint study provided a unique opportunity for independent analysis of different vaccine platforms against Omicron. Adenoviral vector platform in the core of Sputnik V and Sputnik Light vaccines once again proved to be the best solution in creating strong and durable immunity against COVID and its new variants."
Kirill Dmitriev, CEO of the Russian Direct Investment Fund, commented:
"Results of the study in Italy confirm Sputnik V offers the strongest protection against Omicron. The adenoviral platform has shown high efficacy in fighting mutations of COVID previously. Partnership of different platforms is the key and heterologous ("mix & match") boosting with Sputnik Light will help strengthen efficacy of other vaccines in light of combined Delta and Omicron challenge."
Russian Direct Investment Fund (RDIF) is Russia's sovereign wealth fund established in 2011 to make equity co-investments, primarily in Russia, alongside reputable international financial and strategic investors. RDIF acts as a catalyst for direct investment in the Russian economy. RDIF's management company is based in Moscow. Currently, RDIF has experience of the successful joint implementation of more than 100 projects with foreign partners totaling over RUB2.1 tn and covering almost all of Russia's regions. RDIF has established joint strategic partnerships with leading international co-investors from more than 18 countries that total more than $40 bn. Further information can be found at www.rdif.ru
[i] https://www.medrxiv.org/content/10.1101/2021.12.17.21267976v1








![[Today’s K-pop] Blackpink’s Jennie, Lisa invited to Coachella as solo acts](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/21/20241121050099_0.jpg&u=20241121172748)








