Articles by Lim Jeong-yeo

Lim Jeong-yeo
-
[Herald Interview] Carbon-neutral Philips sets higher goals for 2025
For anyone who has tried, it is evident that reducing one’s carbon footprint requires an extraordinary attention to the waste produced, coupled with a conscious effort to cut down on convenient habits that are harmful to the Earth. When the individual effort is enough of a challenge to take on, how does one achieve this seemingly insurmountable task as a company, let alone a global one with international branches? Global health care company Philips has had its eyes on an environmental,
Industry March 1, 2021
![[Herald Interview] Carbon-neutral Philips sets higher goals for 2025](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2021/03/01/20210301000168_0.jpg&u=20210302094204)
-
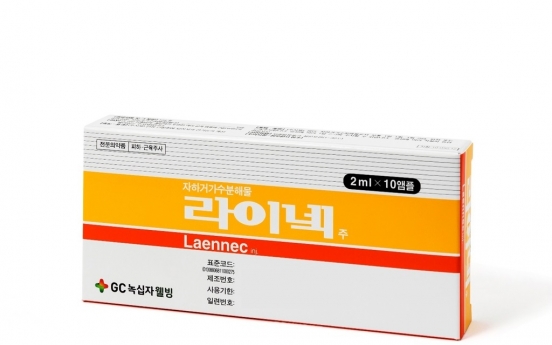
GC WellBeing to repurpose liver drug as COVID-19 treatment
GC WellBeing will conduct a phase 2 clinical trial of its liver drug Laennec, to repurpose it as a COVID-19 treatment, the Ministry of Food and Drug Safety said Friday. This puts the total count of domestic pursuits for COVID-19 treatments at 14 and vaccines at eight. Among them, Celltrion’s monoclonal antibody regdanvimab is the only one to have been emergency-approved. Laennec is made from human placenta. Having been approved in Korea in 2005, the drug has been in use for 16 years a
Industry Feb. 26, 2021
-
Pfizer vaccine approval in Korea imminent, FDA allows more flexible storage
South Korea on Friday paved the way to approving Pfizer’s mRNA vaccine for COVID-19 as another group of pharmaceutical experts positively opined on the vaccine’s efficacy and safety. On the same day, Korea received enough Pfizer vaccines to inoculate 58,500 people, sourced from the global COVAX facility. This lot, under special import exemptions, can be used without the Drug Ministry’s emergency approval, with the first round of vaccinations to start Saturday for frontlin
Industry Feb. 26, 2021

-
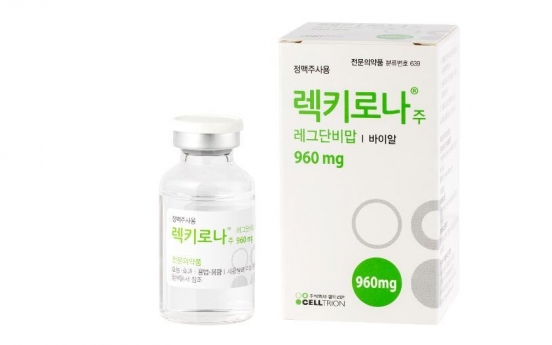
EMA starts rolling review of Celltrion’s COVID-19 treatment
European Medicines Agency said Wednesday, local time, that it has started a “rolling review” of data on South Korean company Celltrion’s monoclonal antibody regdanvimab. Regdanvimab, also known as CT-P59, is being developed by Celltrion for the treatment of COVID-19. A rolling review refers to an on-the-roll evaluation system that accepts data as they come from ongoing research. This form of evaluation is enforced during public health emergency, for a more time-efficient ass
Industry Feb. 25, 2021
-
Christopher Ko shares visions as Bio association representative
South Korean biosimilar maker Samsung Bioepis’ CEO Christopher Ko on Wednesday carried out his first public event as the new chairperson of the Korea Bio Association. Korea Bio is an association representing more than 300 biologics companies in Korea. Ko was made its seventh chief on Jan. 28. He succeeded Macrogen Chairman Seo Jung-sun, who retired from the post after serving five terms. Speaking to reporters online, Ko said that Korea Bio will actively seek to raise homegrown bio compa
Industry Feb. 24, 2021

-
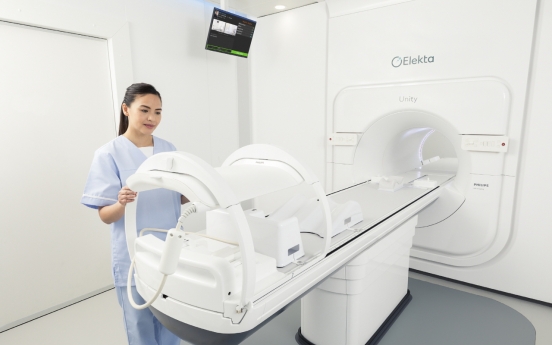
Elekta’s next-gen MRI device offers new hope for cancer patients
Swedish medical device company Elekta wants to give renewed hopes to cancer patients through its next-generation radiotherapy device Unity. Elekta on Wednesday launched Unity in Korea, making it the country’s first 1.5 tesla MRI machine that allows real-time, high-definition monitoring of tumors. Korean cancer patients will be able to access Unity at Yonsei University Gangnam Severance Hospital, starting July 26. “If patients had undergone 20 radiotherapies before, with Unity, the
Industry Feb. 24, 2021
-
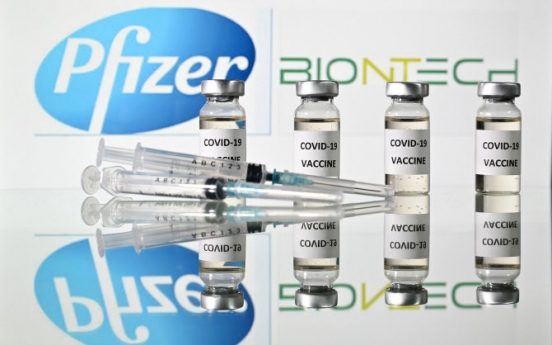
Korean experts advise Pfizer vaccine for 16 and older
The first of the three-part evaluation of Pfizer’s COVID-19 vaccine here has shown the vaccine to be 95 percent effective and eligible for use on those aged 16 and older, South Korea’s Drug Ministry announced Tuesday. The panel of seven, comprising virologists, medical professionals and statistics experts, confirmed that all 36,523 candidates given two shots of the Pfizer vaccine in clinical trials experienced quadrupled antibodies in their system. The panel assessed that the tria
Industry Feb. 23, 2021
-
[Herald Interview] Software dons role of therapeutics
When talking about medical devices, conventional hardware with analytical or therapeutic functions for human health usually come to mind. But computer software that seeks to cure or alleviate symptoms in patients also falls into the medical device category, according to Article 2 of the Medical Devices Act. This new tool, after years of serving an auxiliary role for hardware devices, has recently emerged as a key player in the medical arena. LifeSemantics, a digital therapeutics company foun
Industry Feb. 22, 2021
![[Herald Interview] Software dons role of therapeutics](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2021/02/22/20210222000670_0.jpg&u=20210222183049)
-
Macrogen, Lifex Biolabs to research Parkinson’s treatment
Precision life science company Macrogen will collaborate with US-based Lifex Biolabs on Parkinson’s disease treatment research, Macrogen announced Monday. This is a follow-up to Macrogen’s strategic investment in Lifex in 2020. Under the joint research partnership, Macrogen will sift out biomarkers for Parkinson’s, using its DNA sequence analysis technology. Lifex will use the biomarkers identified by Macrogen to develop Parkinson’s treatments. Biomarkers are tracki
Industry Feb. 22, 2021

-
Samsung Display joins responsible business alliance
Samsung Display’s chief executive officer Choi Joo-sun is aiming to further embolden the company’s environmental, social and governance (ESG) involvement beginning this year. As part of this drive, the company announced Sunday that it joined the Responsible Business Alliance. The RBA is an international industry coalition that holds its members accountable to a code of conduct that aspires for a higher level of corporate ethics. More than 160 global companies are its members, inclu
Industry Feb. 21, 2021

-
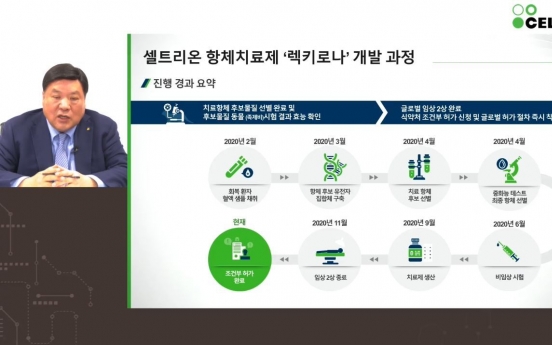
Celltrion to take on S. Africa variant
Only a day after Celltrion began distribution of its monoclonal antibody therapy for COVID-19 in Korea, the company announced Thursday that it is setting out to tackle the more contagious South African variant. “We have a growing library of antibody candidates for COVID-19 variants, of which the ‘32nd antibody’ has shown potency against the South African strain,” said Celltrion’s Honorary Chairperson Seo Jung-jin. “We will enter animal trial of this 32nd a
Industry Feb. 18, 2021
-
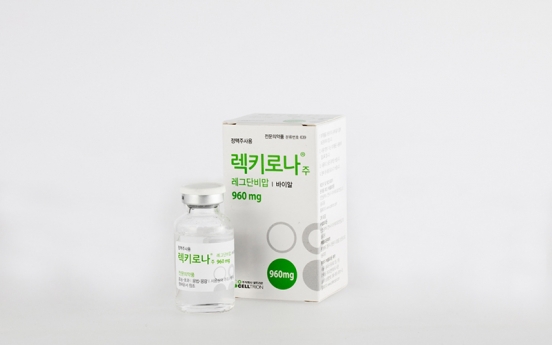
Celltrion begins distribution of COVID-19 therapy in Korea
Celltrion began nationwide distribution of its monoclonal antibody therapy for COVID-19 on Wednesday. Celltrion’s regdanvimab is Korea’s first emergency-approved COVID-19 treatment. All 156 state-designated COVID-19 medical institutes are eligible for free distribution of regdanvimab, Celltrion said, upon individual submission of supply request. Patients who can receive the shot are confirmed COVID-19 patients who have yet to pass seven days from the first onset of symptoms, who
Industry Feb. 17, 2021
-
[News Focus] More homegrown COVID-19 therapies coming
Following Celltrion’s monoclonal antibody treatment for COVID-19, a slew of homegrown therapies is on the way in the first half of 2021. Chong Kun Dang Pharmaceutical is set to submit application for emergency approval in February, and GC Pharma in April. Under the fast-track review program for COVID-19 therapies and vaccines, the new submissions will hear back in 40 days from application. Treatments are primarily for alleviating the condition in afflicted patients, rather than preventi
Industry Feb. 16, 2021
![[News Focus] More homegrown COVID-19 therapies coming](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2021/02/16/20210216000844_0.jpg&u=20210216175226)
-
SK Bioscience to manufacture Novavax vaccine for 20 mil population
South Korean vaccine-maker SK Bioscience will manufacture 40 million doses of US’ Novavax vaccines, enough to inoculate 20 million people. Under the agreement signed by SK Bioscience, Korea Disease Control and Prevention Agency and Novavax on Tuesday, Novavax will license out its NVX-CoV2373 vaccine technology to SK Bioscience for contract manufacturing purposes. As per this deal, South Korea will have the means to stably secure COVID-19 vaccines domestically, without having to rely on
Industry Feb. 16, 2021

-
Celltrion’s Humira biosimilar gains European sales approval
Celltrion’s Yuflyma, an adalimumab biosimilar for autoimmune diseases, has been given the green light by the European Commission on Feb. 11, allowing for it to be sold in the European market, Celltrion announced Monday. Yuflyma is the world’s first Humira biosimilar that is high-concentrate and citrate-free, just the way the original drug was updated and where the rest of the market is headed, Celltrion said. The removal of citrate reduces the pain experienced by patients immediate
Industry Feb. 15, 2021

Most Popular
-
1
Actor Jung Woo-sung admits to being father of model Moon Ga-bi’s child

-
2
Wealthy parents ditch Korean passports to get kids into international school

-
3
Man convicted after binge eating to avoid military service

-
4
First snow to fall in Seoul on Wednesday

-
5
Final push to forge UN treaty on plastic pollution set to begin in Busan

-
6
Korea to hold own memorial for forced labor victims, boycotting Japan’s

-
7
Nvidia CEO signals Samsung’s imminent shipment of AI chips

-
8
Job creation lowest on record among under-30s

-
9
NK troops disguised as 'indigenous' people in Far East for combat against Ukraine: report

-
10
Opposition leader awaits perjury trial ruling


![[Herald Interview] Carbon-neutral Philips sets higher goals for 2025](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2021/03/01/20210301000168_0.jpg&u=20210302094204)






![[Herald Interview] Software dons role of therapeutics](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2021/02/22/20210222000670_0.jpg&u=20210222183049)




![[News Focus] More homegrown COVID-19 therapies coming](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2021/02/16/20210216000844_0.jpg&u=20210216175226)











